Abstract
In March 2020, the World Health Organization declared COVID-19 infection a pandemic. The management and treatment plan have been based on previous evidence on use during viral pandemics such as Spanish flu, Ebola Virus, Middle East Respiratory Syndrome.
SARS-CoV-2 presents a lot of complications as the disease progresses and most of these complications contribute to the mortality of the patients with approximately 7% of the reported deaths being attributed to myocarditis and more deaths attributed to the hypercoagulable state secondary to the infection.
This paper focuses on myocarditis, clotting abnormalities and acute kidney injury complications developed secondary to infection with the virus and the current management practices for patients with these complications.
Keywords: COVID-19, Complications, Myocarditis, Clotting abnormalities, Acute Kidney Injury
Background
SARS-CoV-2 has affected different races, genders and ages differently, making it difficult to establish a standard global management protocol. The symptoms can be mild to moderate where there is no pneumonia development and the patients may be managed from home with the right protocols followed. It may progress to severe where the patient needs hospitalization and the patients showing symptoms of dyspnea, hypoxia and lung involvement. In the critical stage, complications are pronounced and there is respiratory failure and multi-organ failure[1].
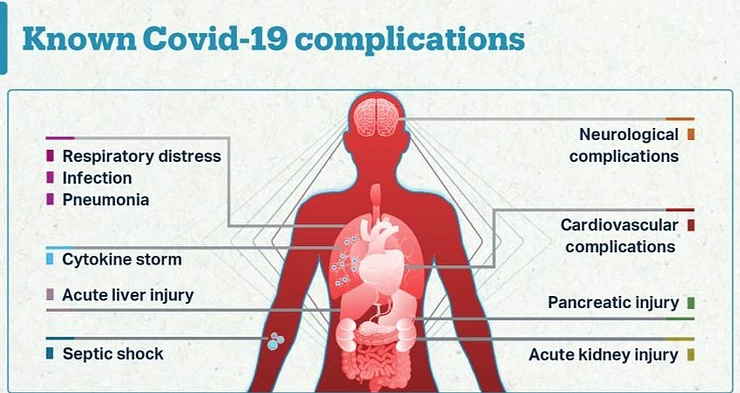
Currently, the identified complications are; neurological such as acute necrotizing encephalopathy and loss of smell and taste; cardiovascular such as myocarditis, myocardial injury, myocardial infarctions, acute heart failure and cardiomyopathy; hematological abnormalities including hypercoagulable state; respiratory complications such as respiratory failure; renal complications such as kidney failure and dermatological complications such as exanthema and pruritis[2].
There are conventional management plans for the patients, however, they vary depending on their efficacy and inter-individual variations[3].
Pathogenesis
SARS-CoV-2 virus primarily enters the body through the nose when air with saliva droplets containing the virus are inhaled[4]. The virus can also enter the body through the mouth and eyes which forms the basis for infection prevention and control guidance on avoiding touching of faces. When the virus enters the body, it attacks the upper respiratory tract resulting in classical respiratory symptoms of dry cough and difficulty breathing. The epithelial cells on the oral and nasal mucosa, nasopharynx, lung, stomach, intestine, liver, kidney and brain have angiotensin converting enzyme-2 (ACE-2) receptor on their surface[5]. The SARS-CoV-2 virus envelope has S1 protein spikes and uses it to bind onto the enzymatic portion of the ACE-2 receptor and the serine protease enzyme cleaves the peptide bonds on the S1 protein resulting in endocytosis and translocation of the virus and the ACE-2 enzyme into the cell. In the cells it replicates, colonizes the cell and kills the cell. The replicated virus then spreads to affect the neighboring cells.
It infects the type II alveolar cells in-charge of surfactant production in the lungs and when they die, they release cytokines: Interleukin 1, Interleukin 6 and Tumor Necrosis Factor α which cause inflammation of the surrounding tissues and the neighboring blood vessels and their high concentration result in a cytokine storm. Death of the Type II cells increases surface tension in the Type I alveolar cells making them to collapse causing shortness of breath and hypoxia. These cytokines are chemotactic factors that attract neutrophils which kills the virus with Reactive Oxygen Species and proteases, however, in the process more alveolar are destroyed, increasing the hypoxic state. The inflammation of the neighboring blood vessels results in vasodilation and increased capillary permeability resulting in alveolar edema which contributes to the shortness of breath as the alveolar are filled with fluid.
Clotting Abnormalities
In Italy 22.2% of reported COVID-19 patients and 14.7% of 184 Dutch patients were diagnosed with venous thromboembolism. 16.7% of 150 French patients developed pulmonary embolism despite prophylactic antithrombotic therapy. 69% of the COVID-19 patients in ICU under critical conditions have severe clotting complications that contributes to most of the deaths.
The etiology of thromboembolisms in these patients is not clearly defined. Normally, thrombin levels in blood are highly regulated with endogenous antithrombin III, protein C system and tissue factor pathway inhibitor. Inflammation in the systemic circulation alters the control system and reduces the antithrombin levels resulting in formation of microthrombi in the blood vessels and increased inflammation through the proteinase activated receptors (PAR). Hypoxia secondary to dyspnea and systemic inflammation due to inflammatory cytokines activate the coagulation pathway increasing clot formation[6]. Hospitalized patients are always in bed and this is also a predisposing factor to clot formation. Laboratory tests of COVID-19 patients indicate mild thrombocytopenia, prolonged prothrombin time and increased D-dimer, fibrin, fibrinogen and fibrin degradation products levels. Patients with high sustained levels of the latter four mostly die. Other clear indications of clotting abnormalities include microvasculature thrombosis of the toes, clotting on catheters and large vessel strokes.
Low molecular weight heparin inactivates the activated Factor X, inhibits the conversion of prothrombin to thrombin, prevents the activation of fibrinogen to fibrin and prevents the formation of a stable fibrin clot by inhibiting fibrin stabilizing factor. It is preferred when the patient has high d-dimer levels. It also reduces inflammation. Warfarin antagonizes Vitamin K epoxide reductase enzyme hence the active form of Vitamin K is unavailable to carboxylate factor VII, IX, X and thrombin. These two are used for prophylaxis and treatment of deep venous thrombosis, venous thromboembolism and pulmonary thrombosis. Oral anti-factor Xa are used to reduce the proinflammatory cytokines in the blood and PAR-1 antagonists are used to reduce levels of proinflammatory cytokines and neutrophil lung inflammation[7].
Patients with underlying conditions requiring antithrombotic therapy are encouraged to continue with their doses as per their doctor’s recommendations. Patients who are undergoing home treatment are not allowed to self-administer antithrombotic agents unless it is verified. Adults and the elderly diagnosed with COVID-19 and are hospitalized are required to take venous thromboembolism prophylaxis and should undergo frequent laboratory screening to monitor their condition. Arterial thrombosis should be managed with appropriate medication and not anticoagulants meant for venous thromboembolism.
Therapy is recommended for high risk patients with a low risk of bleeding but a high risk of developing thromboembolisms. Lactating mothers can be given unfractionated heparin, low molecular weight heparin because it does not accumulate in breast milk, it has a short half-life and has fewer drug-drug interactions. Pregnant women should continue with their prophylaxis dose because they are already predisposed to developing clots. Warfarin is equally recommended to patients with mechanical heart valves, ventricular assisting devices, anti-phospholipid antibody syndrome and valvular atrial fibrillation.
Myocarditis
Myocarditis is characterized by inflammation of the heart. SARS-CoV-2 affects the myocardium through the Angiotensin Converting Enzyme 2 (ACE2) receptors on the myocytes. Inhibition of these enzymes increase angiotensin 2 levels which is pro-inflammatory. The aggressive immune response to the virus in the systemic circulation and the overproduction of proinflammatory cytokines secondary to activation of the coagulation pathway causes cardiomyocyte dysfunction increases inflammation in the heart resulting in myocarditis.
Laboratory findings include increased serum alanine aminotransferase, lactate dehydrogenase and aspartate aminotransferase levels. C-reactive proteins and troponin I levels are also high. Out of 41 patients in Wuhan, 12% of them had increased troponin I levels. Out of 1527 randomly sampled patients, 16.4% developed cardiac diseases secondary to SARS-CoV-2 infection and 22-31% of those who ended up in the ICU developed myocardial injury and myocarditis attributed to the high viral load and mononuclear infiltration in the myocardium.
Management of myocarditis requires careful selection of medication and constant patient monitoring. In hypotension aggressive fluid replacement should be avoided and adrenaline then dobutamine can be used to stabilize the patient[8]. Methylprednisolone is an immunomodulator. It binds onto nuclear receptors in the myocytes and inhibits pro-inflammatory signals and promotes anti-inflammatory signals. However, it interacts with some anti-arrhythmic drugs and increases fluid retention in the body and may lead to hypertension. Intravenous immune globulin is also used to reduce inflammation. It is immunomodulatory and works by reducing the production of antigen-specific T cells, leaving them in a refractory stage after which apoptosis is induced by CD95[9]. Tocilizumab inhibits IL-6 binding onto its receptor. Inhibition of IL-6 signaling prevents recruitment of inflammatory cells B and T cells, reducing inflammation. Anakinra is an antagonist to IL-1 receptor and it reduces the immune response. Antivirals such as Lopinavir/Ritonavir are used in severe conditions to reduce the viral load which worsens myocarditis. Lopinavir inhibits the 3-chymotrypsin-like cysteine protease enzyme, inhibiting cleaving of the polyproteins resulting in the production of immature non-infectious viral particles. Ritonavir inhibits the enzymes that metabolize Lopinavir, increasing the duration of action. However, their dangerous side effects such as prolonging QT and PR interval, it causes AV block and is associated with drug-drug interactions with anticoagulants.
Acute Kidney Injury
Approximately 40% of the patients in the ICU in the USA and in Europe have developed acute kidney injury. The SARS-CoV-2 virus directly affects the renal artery endothelium through the ACE-2 receptors resulting in microthrombi and micro-embolism which results in kidney infarction[10]. It also affects the podocytes with ACE-2 receptors, damaging the epithelial cells of the Bowmans Capsule and filtration process is hindered resulting in proteinuria. The renal tubular epithelium is also damaged resulting in mitochondrial dysfunction and acute tubular necrosis. Pneumonia secondary to COVID-19 infection causes right ventricular failure that congests the kidney, damaging it. Left ventricular dysfunction causes low cardiac output and atrial underfilling hence kidney hypoperfusion leading to acute kidney injury[11].
Laboratory indications include hyperkalemia, metabolic acidosis, oliguria and uremic encephalopathy. The laboratory markers should be monitored to detect injury early and provide the best management. Fluid balance should be adjusted to avoid an overload that will worsen alveolar edema. Patients with hypovolemia must also be given fluids. Early continuous renal replacement therapy (CRRT) and extracorporeal organ support improves the patient’s condition and prevents the development of acute kidney injury. The right jugular vein is the most preferred vessel for CRRT[12].
Anticoagulants must be administered when employing CRRT and extracorporeal organ support. Citrate anticoagulants are the most preferred and the doctor must tailor the dose to the patient’s individual requirements. LMWH, unfractionated heparin and systemic anti-factor Xa can be used with an initial dose of 3.5mg/h, 10-15IU/kg/h and 0.25-0.35 IU/ml is recommended. LMWH is preferred over unfractionated heparin because it gives a better anticoagulation effect before filtration. The disadvantage of using heparin is the risk of hemorrhage, resistance and heparin induced thrombocytopenia[13].
Calcium concentration after the filter is reduced to improve the patency of the filter. Continuous Veno-Venous Hemodialysis modality (CVVHD) increases cytokine removal, reducing kidney injury from the cytokine storm. This form of therapy is applied in immune system dysregulation where all other supportive methods are not working sufficiently.
Conclusion
COVID-19 related complications are associated with multi-organ dysfunction and systemic inflammation. Without a definite treatment, supportive treatment is needed to reduce the pain and to fasten the recovery of the patients. The medication used for the management of myocarditis and clotting abnormalities should be closely monitored because many of them interact and compromise recovery. Thromboembolism prophylaxis is a compulsory guideline to prevent severe clotting abnormalities. Acute Kidney Injury has to be detected early with the laboratory markers to reduce the injury as much as possible. The prevailing condition of the patient determines the best approach in managing acute kidney injury. Hemodynamically unstable patients are managed with CRRT while patients with immunity dysfunction states are managed with CVVHD.
[1] Interim Clinical Guidance for Management of Patients with Confirmed Corona Virus: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html [2] Cardiovascular Complications in COVID-19: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7165109/ [3] NIH COVID-19 Treatment Guidelines: https://covid19treatmentguidelines.nih.gov/whats-new/ [4] How does the coronavirus enter the body, and what makes it so dangerous? https://www.timesofisrael.com/how-does-the-coronavirus-enter-the-body-and-what-makes-it-so-dangerous/ [5] Tissue Distribution of ACE-2 Protein, the functional receptor for SARS Coronavirus. The First Step in Understanding SARS Pathogenesis: https://pubmed.ncbi.nlm.nih.gov/15141377/ [6] COVID-19 and Coagulopathy: https://www.hematology.org/covid-19/covid-19-and-coagulopathy [7] COVID-19 cytokine storm: the interplay between inflammation and coagulation : https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(20)30216-2/fulltext [8] Management of the Hospitalized COVID-19 patients with Acute Cardiomyopathy of Heart Failure: https://www.acc.org/latest-in-cardiology/articles/2020/04/16/14/42/management-of-the-hospitalized-covid-19-coronavirus-2019-patient-with-acute-cardiomyopathy-or-heart-failure [9] Clinical Use of Intravenous Immunoglobulin: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1809480/ [10] Corona Virus: Kidney Damage Caused by COVID-19: https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/coronavirus-kidney-damage-caused-by-covid19 [11] Management of acute kidney injury in patients with COVID-19: https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(20)30229-0/fulltext [12] Advice For Managing Acute Kidney Injury In COVID-19 Patients: https://www.medscape.com/viewarticle/929275 [13] Anticoagulation for continuous renal replacement therapy: https://pubmed.ncbi.nlm.nih.gov/19426417/
Author: Ms. Mayuba R. Michele